 By Luke O’Donnell, MD
By Luke O’Donnell, MD
Peer reviewed
Once formidable diseases, pneumonia, bacteremia, and meningitis are all now considered “bread-and-butter” internal medicine. Streptococcus pneumoniae is one of the major …

 By Luke O’Donnell, MD
By Luke O’Donnell, MD
Peer reviewed
Once formidable diseases, pneumonia, bacteremia, and meningitis are all now considered “bread-and-butter” internal medicine. Streptococcus pneumoniae is one of the major …
 By Pritha Subramanyam
By Pritha Subramanyam
Peer Reviewed
Mrs. CS is a 66-year-old Indian female who presents for a cardiology follow-up. The patient has a history of mitral regurgitation secondary to rheumatic fever …
 By Theresa Sumberac, MD
By Theresa Sumberac, MD
Peer Reviewed
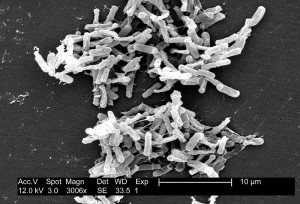
Antibiotic associated diarrhea is a common complication of antibiotic therapy, occurring in 5% to 39% of all patients receiving treatment. Nearly one third of these cases …
 By Richard E. Greene, MD
By Richard E. Greene, MD
Peer Reviewed
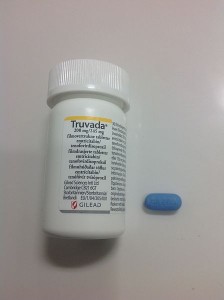
In July of 2012, the FDA approved the use of Tenofovir-Emtricitabine (Truvada, a single blue pill) daily as Pre-Exposure Prophylaxis …
 By Aaron Smith, MD
By Aaron Smith, MD
Peer Reviewed
It’s become a familiar site to travelers: airline passengers wearing respiratory masks to filter pathogens from the cabin air. To those not wearing masks, the …
 By Aaron Smith, MD
By Aaron Smith, MD
Peer Reviewed
First introduced in the late 1980s, proton pump inhibitors (PPIs) have revolutionized the treatment of gastric acid-related disorders and have been described as a miracle drug …
 By Julian Horwitz
By Julian Horwitz
Peer Reviewed
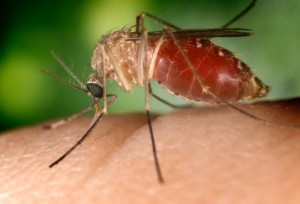
As of mid-August 2012, the CDC had reported 1118 cases of West Nile virus (WNV) infections and 41 related deaths, which, pro rata, made 2012 the most …
 By Aaron Smith, MD
By Aaron Smith, MD
Peer Reviewed
Case: A 35 year-old, overweight female presents to the emergency room with five days of left lower quadrant abdominal pain. The pain is 10/10 in …