 By Daniel Gratch, MD
By Daniel Gratch, MD
Peer Reviewed
In 150 AD, Greek physician and philosopher Galen wrote of a woman suffering from insomnia: “I was convinced the woman was afflicted not by a bodily disease, but …

 By Daniel Gratch, MD
By Daniel Gratch, MD
Peer Reviewed
In 150 AD, Greek physician and philosopher Galen wrote of a woman suffering from insomnia: “I was convinced the woman was afflicted not by a bodily disease, but …
 By Chio Yokose, MD
By Chio Yokose, MD
Peer Reviewed
Even in this era of modern medicine, bacterial meningitis remains a widely feared diagnosis in both resource-rich and -poor settings worldwide. Bacterial meningitis is among the ten most common …
 By Rebecca Sussman
By Rebecca Sussman
Peer Reviewed
Reviewing medical evidence has become such a habit that sometimes it feels almost impossible to think independently. I’ve always been a top-down thinker; I go with my …
 By Joseph Zakhar
By Joseph Zakhar
Peer Reviewed
The Patient:
Fate is the sound of a ringing phone.
I, however, am growing to hate the sound.
I’m strangled by the words, by the rough sheets, the …
 As we reach the end of another year, we want to wish all our readers a very happy and healthy New Year. And not to …
As we reach the end of another year, we want to wish all our readers a very happy and healthy New Year. And not to …
 Nicholas Mark, MD & Sarah Buckley, MD
Nicholas Mark, MD & Sarah Buckley, MD
Faculty Peer Reviewed
Background
Publius Aelius Hadrianus, better known as Hadrian, emperor of Rome (117-138 CE), traveler, warrior, and lover of all things Greek, fell ill at the age of 60. He developed progressive edema and …
 By David Ellenberg
By David Ellenberg
Faculty Peer Reviewed
Mr. A is a 91 year old male with a history of hypertension and two myocardial infarctions. He presented with shortness of breath and worsening lower extremity swelling and …
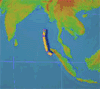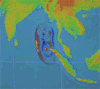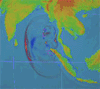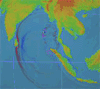 By Irene Isabel Payad Lim, MDÂ and Michael Ford, MD
By Irene Isabel Payad Lim, MDÂ and Michael Ford, MD
Faculty Peer Reviewed
The February 27, 2010 earthquake in Chile measured 8.8 on the Richter scale and displaced nearly …